Agronomic Insights

Author: Lee Menhenett – Technical Agronomist
Achieving even, rapid crop emergence is fundamental to maximising paddock yield. Several factors can hinder seedling germination and vigour, including incorrect seed placement (either too shallow or too deep), inconsistent sowing depths across the bar, soil throw into furrows, and incorrect configuration of tyne or disc fertiliser placement. Addressing these issues is critical to ensuring optimal crop establishment.
While adequate sowing moisture can mask some plant establishment challenges, there will be a period during sowing where moisture will be limiting. Also, spatial variation in soil type across a paddock can complicate seeder tyne settings. The point being that some level of seed loss is inevitable, reducing overall germination rates. However, by understanding these risks and putting management strategies in place, growers can minimise crop losses and reduce the need for costly reseeding operations.
Fertiliser placement is another key consideration. Placing phosphorus (P) and nitrogen (N) near or with the seed enhances early plant vigour and root development. However, improper placement can have significant negative consequences. Fertiliser toxicity can delay or reduce emergence, primarily due to high ammonia concentrations or osmotic stress (‘droughting’), particularly when excessive N is applied at seeding. Other influencing factors include crop type, soil moisture levels, soil characteristics, and the precision of application equipment.
To mitigate these risks, calculating the Seed Bed Utilisation (SBU%) is essential. This metric helps determine the appropriate fertiliser rate before planting, ensuring optimal nutrient availability while minimising seed damage and establishment failures.
Know your fertiliser
The osmotic effect of a fertiliser on seed during germination is primarily related to the chemistry of the fertiliser and the solution concentration (salt index).
Where moisture is limited, the fertiliser granule may compete with the seed for moisture. The osmotic effects on germination are usually shown as delayed or staggered emergence, rather than complete failure of the crop.
Fertilisers containing ammonium can cause metabolic damage during germination, root or shoot growth. Fertilisers such as urea, di-ammonium phosphate (DAP), mono-ammonium phosphate (MAP) and ammonium sulphate pose the greatest risk to a germinating seed because they produce a high ammonium concentration and have a high solution pH and high solubility.
As urea breaks down, it produces ammonium carbonate which causes alkaline conditions around the fertiliser granules. The change in pH around the urea granule results in the production of ammonia gas which is highly toxic to germinating seeds.
The hierarchy of risk is: urea > DAP > MAP > Ammonium Sulphate. If using a MAP/urea blend at planting remember there is a higher risk with the inclusion of urea.
Figure 1: pH and effect on Ammonium/Ammonia balance.
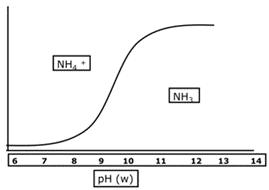
Source: Incitec Pivot
Most crop damage occurs in the week following sowing, when ammonia production and salt osmotic effects kill the seed or the germinating seedling. While P toxicity is generally of less concern, the issue needs some consideration particularly for sensitive crops like canola and lupins.
Trial work from Western Australia suggests toxicity from phosphate is less on soils with a lower phosphorus status and with higher P fixing capacity (PBI), (Mason et al. 2006).
Image 1: Albus lupins at Incitec Pivot Fertilisers’ Grenfell long-term trial site in 2010. The nil P with the seed is the plot on the left, showing better establishment and vigour than the 10 kg/ha of phosphorus as triple superphosphate applied with the seed in the plot on the right.

Crop type
Winter crops vary in their tolerance to fertilisers, with oats, barley and wheat more tolerant than lupins and chickpeas. Canola is the most sensitive winter crop type. Larger seed sizes have smaller surface areas compared to small seeds, decreasing the area of seed exposed to ammonia or salt from fertiliser. Small seeds also have smaller root and shoots that tend to be more prone to damage.
Image 2: Significant reduction in plant population due to urea placement with the seed at the IPF long-term trial site at Grenfell 2012.

External environment
Soil moisture has a major bearing on the effect of fertilisers in contact with the seed. In seasons with high soil moisture levels at planting, crop damage is generally not an issue. Soil moisture provides soil tilth, this allows good soil/seed contact, minimising air pockets around the seed where ammonia gas can move into. Soil moisture buffers the ammonia and salt effect of the fertiliser, effectively providing a barrier against and dilution of gas and salt movement.
The rate of drying can be as important as initial soil moisture levels during the germination and establishment phase. If soil moisture levels fall post-sowing, the potential for reduced emergence will increase. The sowing operation causes the row to dry more quickly due to the introduction of air, therefore marginal soil moisture at sowing instantly becomes problematic.
Rain events post-sowing will dilute salt and ammonia concentrations around the fertiliser.
Time of sowing can also affect crop emergence. Early sowing into warm, moist soils results in quicker germination and crop emergence which reduces the amount of time the seed is in contact with the fertiliser in the seed row.
Soils
Fertiliser applied at any rate on lighter sandy soils carries a greater risk of seedling damage than on heavier clay soils. Clay soils have a higher cation exchange capacity which are better able to retain water and adsorb ammonium. Texture also influences the rate of water and gas movement, therefore providing greater buffering for the seed and seedling. Sowing into heavier soil types still requires consideration particularly when moisture is marginal, and soil tilth is poor. Clods and lack of row closure causing air pockets around the seed can cause significant seed loss.
Fertilisers can further dry out soils (osmotic effect) around the seed by absorbing moisture. This reduces the viability of the seed. In dry autumns and when planting into marginal moisture, it is common to see the negative impact that low soil moisture and fertilisers can have in reducing plant population and vigour.
Application equipment
The safest application equipment allows the fertiliser and seed to be applied in separate bands.
Applying fertilisers below (25 mm) and to the side of the plant line (25 mm) is the most effective method of avoiding seed or root contact in winter crop. Higher rates of N may need greater separation.
Banding N-based fertilisers directly below the seed can allow ammonia gas to move through the tyne/disk slot directly to the seed. Therefore, if fertiliser can’t be offset then at least 3 cm separation is required (greater separation in lighter soils). Excluding N may be a better choice where soil closure cannot be achieved due to clodiness or tyne set up when placed directly below the seed.
Minimum till sowing equipment has significantly decreased furrow widths. By decreasing furrow widths there is less soil mixing with the fertiliser and seed, increasing the potential of harmful seed and fertiliser interactions. Any change in application equipment should be considered for its potential to increase or decrease seed safety.
Row spacing significantly influences the concentration of fertiliser because there are less metres of row per hectare. There is more than one grower who has learnt the hard way that increasing the row spacing and keeping fertiliser rates the same can result in reduced plant population.
Further information
For more information, contact Lee Menhenett on 0412 565 176 or lee.menhenett@incitecpivotfertilisers.com.au
References
Mason MG, Jarvis RJ, & Bolland MDA. (December 2006). Fertiliser toxicity and crop establishment in no-tillage farming. Crop Establishment Series, Farmnote 72/96.
Further reading
M and O’Brien B (2021) Optimising canola establishment and performance by phosphorus fertiliser placement. GRDC Update Paper. Grains Orana Alliance
Dockerill J, McLaughlin M and Degryse F (2023) Developing an ecotoxicologically based index for fertiliser toxicity. GRDC Update Paper. University of Adelaide.
Norton R and Desbiolles J (2011) Fertiliser Toxicity Fact Sheet. GRDC.
DISCLAIMER
This is a guide only, which we hope you find useful as a general tool. While IPF has taken all reasonable care in the preparation of this guide, it should not be relied on as a substitute for tailored professional advice and IPF accepts no liability in connection with this guide. Incitec Pivot Fertilisers manufactures and sources fertilisers from other suppliers. The fertiliser supply chain extends beyond the company’s direct control, both overseas and within Australia. Incitec Pivot Fertilisers hereby expressly disclaims liability to any person, property or thing in respect of any of the consequences of anything done or omitted to be done by any person in reliance, whether wholly or in part, upon the whole or any part of the contents of this article.
You might also be interested in these

Horticulture, Pasture, Sugar, Summer Crop, Winter Crop
Soil sampling for reliable results
February / 2025

Sugar
A holistic approach to minimise nitrogen loss
July / 2023

Horticulture, Pasture, Sugar, Summer Crop, Winter Crop
Get your coat on
October / 2024

Horticulture, Pasture, Sugar, Summer Crop
Take the next step with leading Agronomy in Practice course
February / 2024

