Agronomic Insights

Fiona McDonald – IPF Technical Agronomist
Potassium plays a pivotal role in crop success, but its influence is far from simple. In this article, we discuss the complexities of potassium. From understanding its distribution in soils to decoding deficiency symptoms, we discover the potential of potassium and tools to assist when recommending it.
Potassium:
- Vital in many essential functions.
- Removal is varied across operations.
- Soil type may affect the way it’s held, or isn’t (leaching)
- Importance of soil and plant tissue testing.
- Application of K
Potassium (K) is one of the three major nutrients required in relatively large quantities for the soil to be productive. Unlike nitrogen and phosphorous, K is not as well understood, particularly its behaviour in the soil, root physiology, testing for K and fertiliser application.
Potassium is highly mobile within the plant and is vital in many essential functions. It helps with regulation of water pressure in plant cells, enzyme activation, photosynthesis, starch formation and protein synthesis, and fruit initiation. One of its primary roles is its function in stomatal control, which can be associated with some drought tolerance.
To illustrate the disparity in nutrient removal between various agricultural operations, refer to Table 1. The potassium requirement varies significantly when comparing the practice of removing stalks during hay cutting to that of harvesting grain.
| Crop | N (kg/t) | P (kg/t) | K (kg/t) | S (kg/t) |
| Vetch Hay | 18.9– 32.4 | 1.8-2.3 | 16.2 -19.8 | 1.2-2 |
| Oaten Hay | 8.5-10.8 | 0.9-1.4 | 9 – 15.3 | 0.6-0.9 |
| Barley Grain | 14.4-18.9 | 2-2.9 | 3.8 – 5 | 1-1.3 |
| Wheat Grain | 15.3-23.4 | 2.3-4 | 3.7 – 5.3 | 1.2-1.9 |
Soils contain a large amount of potassium, however only a small amount of it is available for plant uptake. Potassium must be present at the surface of the root for it to be taken up by the plant. Potassium can reach the surface of the root in three ways: root interception, mass flow and most importantly diffusion.
Diffusion is the main way plants acquire K and it works by creating a concentration gradient within the immediate vicinity of roots. The plant takes up K, which then decreases the concentration near the root, allowing potassium to move towards the root. This is a relatively slow process requiring water, so can be further slowed by dry conditions.
When K levels are high in the soil this process can be sped up. This can also be due to the plants ability to take up ‘luxury amounts’ – an increase in the plant tissue level, but not in biomass. As plants don’t often show a toxicity effect from excess levels of potassium, it may only lead to an inefficiency in the use of fertiliser. In some situations, it may lead to other difficulties (e.g. reduce uptake in other cation nutrients such as magnesium).
Once the potassium is within the plant it is very mobile, therefore deficiency symptoms are generally seen in older parts of the plant. Symptoms are best described as leaf scorch, firing or spotting. This develops from an initial yellowing of interveinal areas near the leaf margins, and is followed by tanning and browning, and finally drying of the tissue to appear as scorching. Plants that are deficient may tend to grow more slowly, have weaker stalks, poorly developed root systems and small leaves. These may be observed in the field with an increase in the incidence of lodging, frost, or disease, which can demonstrate the spatial variation (difference across areas in paddock or farm) of potassium.
Generally once symptoms have been discovered it’s too late to manage the deficiency in the current season. Instead, it’s a case of planning ahead for next season by completing a soil test and applying adequate fertiliser.
Potassium within the soil
Outside of adding more potassium to the system with K Inputs (fertiliser, manure, crop residue) and losses (leaching and erosion), there are four commonly known ‘pools’ of potassium that interact and cycle to maintain the equilibrium of K within a soil.
The rate of conversion between the pools depends on various factors such as root uptake, fertiliser application, soil moisture and temperature. The four pools are:
- Structural, >90% – unavailable for plant uptake. K is held within the crystal lattice of primary and secondary minerals e.g., micas and feldspars.
- Fixed/Interlayer in secondary minerals K, 1-10% – slowly available and is held between crystal lattice layers.
- Exchangeable K, 1-2% (90% of available) – held on exchange sites, and available for uptake.
- Solution K, 1-2% (10% of available) – available for plant uptake. Dissolved in water (water soluble).
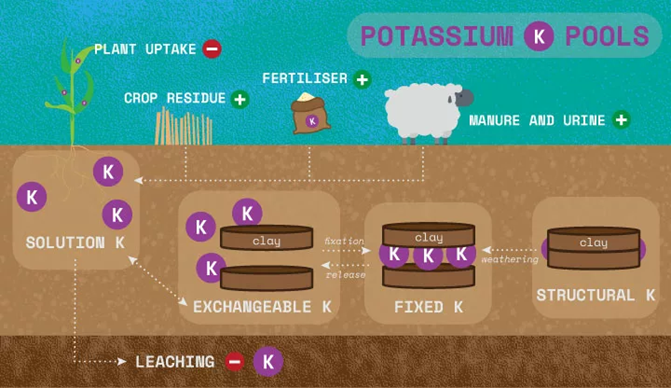
Source: https://soilswest.org.au/under-the-radar-potassium-on-loamy-soil/
Soil type affects the way potassium is held within the soil. For instance, if you have a heavier clay soil, K can be more tightly held and is not as prone to leaching, compared with a sandy soil. Rainfall also accentuates nutrient loss through leaching, and these factors need to be considered to optimise productivity potential.
Plant physiology
The depth at which plant roots reach and access potassium will impact the nutrient level at varying depths. The type of species you’re growing, whether it is tap-rooted or a fibrous system, will give you an indication of depth at which the plant is accessing and potentially depleting K reserves. For example, tap-rooted species such as lucerne can access deeper horizons, compared with plants with a fibrous system such as wheat. Of course, soil moisture will play apart in this, if the topsoil becomes drier and the water-soluble nutrients are no longer available the roots will go searching deeper.
This was supported by the research conducted by Witter and Johansson (2001). In their study, they conducted a comparative analysis of Lucerne and Ryegrass, both cultivated under identical conditions but with distinct root systems: Lucerne had a tap-rooted system, whereas Ryegrass had a fibrous root system. Their findings revealed a significant disparity of potassium uptake in the sub soil. Lucerne was able to obtain about 67% of its K from the subsoil, while ryegrass only obtained 42% of its K from the same source.
K inputs
Crop residue can return a large amount of potassium to the soil and the amount of K in the straw compared to grain is significant. Research has shown that up to 50-60% of K accumulated in straw is released within 40-45 days from plant desiccation, and rainfall or irrigation will drive the K release processes (C. A. Rosolem et al). This can be a common field observation whereby there is a notable difference with the placement of crop residue in windrowing.
With both crop residue and fertiliser applied to the topsoil it can become concentrated or stratified at or near the soil surface but remain low at depth. This may result in plants not getting enough potassium when the surface layers of soil dry out and the roots are drawing soil moisture and feeding for nutrients from deeper in the soil profile.
Soil sampling K
Commonly 0-10cm soil samples are taken in pasture and cropping systems to monitor and diagnose limiting factors. However, if there is a potential K deficiency, and with our understanding of the movement of potassium combined with root physiology we should start to think about taking subsoil samples at 10-30cm depth, to gain an understanding of levels further down the soil profile.
Potassium tests commonly used in Australia are either exchangeable K, using ammonium acetate and Colwell K. These two tests measure the soluble/plant available K and the exchangeable K pools in low fixing soils. In soils with multi-layer clay minerals it may be useful to undertake a soil test that measures both the size of the fixed K pool and its rate of release, such as nitric acid or sodium tetraphenylborate extraction.
| Low | Marginal | Optimum | High | |
| All soils | < 40 | 40 – 79 | 80 – 120 | >120 |
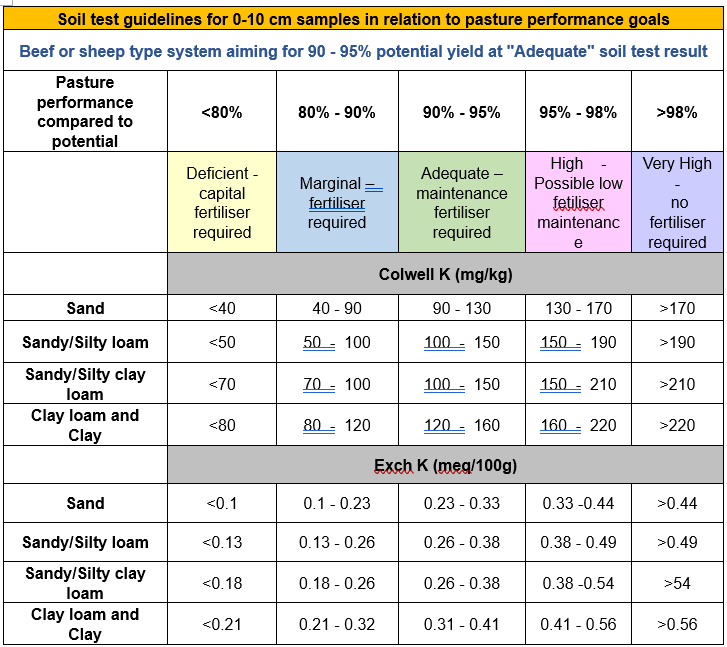
Table 3. Pasture soil test guidelines – Potassium (Colwell K, and Exch K)
Source: Department of Primary Industries, Victoria
Plant tissue
If a potassium deficiency is suspected based on observation of windrowing effect or legume response in urine patches, or plants showing deficiency symptoms, a plant tissue test is a great method to confirm whether the soil could be responsive to K.
An IPF trial was completed looking at K levels within a paddock that displayed windrowing effect (Figure 2). Both soil and plant tissue tests were taken within and between rows, and grain samples at harvest, results shown in Table 4 & 5.

Figure 2. Paddock that displayed windrowing effect

Figure 3. Visual potassium deficiency symptoms in the between row of trial
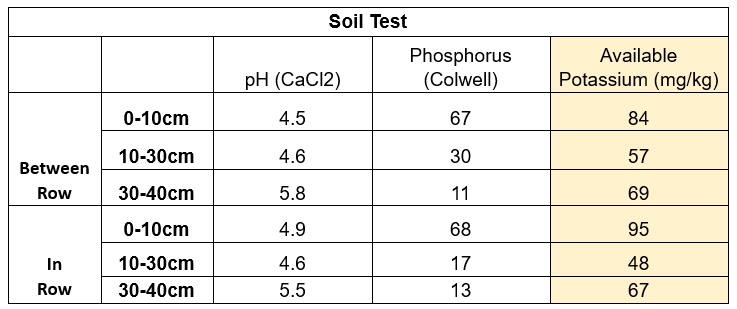
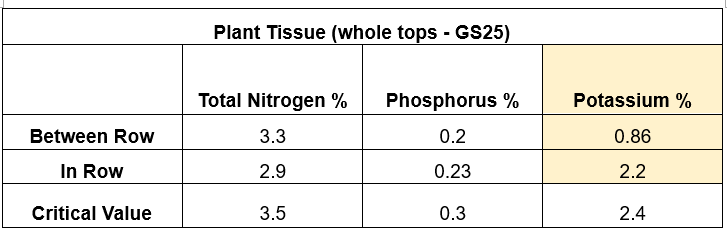
Looking at the results, there were not large differences across the soil samples, however there were in the tissue samples. This trial was then harvested, and the average yield determined (t/ha), 6.87 and 8.88, between the row and in the row, respectively. It is important to note that a yield response is not always achieved when applying capital applications of K, due to the complexities around potassium management, as previously discussed.
A plant tissue test can be a useful tool in diagnosing a potassium deficiency. Critical values vary across species and age of plant – it is critical to follow the correct sampling guidelines to get an accurate interpretation.
Application
When completing a fertiliser recommendation it is important to address the most limiting factor to ensure you get the most economical production responses.
Once added to the soil, fertilisers containing potassium (organic, synthetic, or crop residues, etc.), will end up supplying the same form of potassium. Once it enters the potassium cycle there are a several paths it may go, depending on various factors including soil characteristics, plant requirements, environment, and application rate.
There are two common potassium fertilisers – 1) Muriate of Potash (MOP)/Potassium chloride (KCl), and 2) Sulphate of Potash/ Potassium Sulphate (SOP). MOP is most widely used due to being most economical per unit of K, however it’s not always suitable for all situations with its chloride content. A crops’ sensitivity must be considered along with the potential to further exacerbate a salinity issue. It is therefore recommended to be spread before or at seeding in a band near, but not with, the seed because as it dissolves, the salt concentration increases which may cause damage to the seedlings due to the osmotic potential.
When determining rates, we should be looking at the current soil level to determine if we need capital application to build soil fertility, or a maintenance rate is needed to replace removal. Other considerations that should be looked at in a pasture situation are potential animal health issues.
Due to potassium’s interaction with calcium and magnesium, it can cause a deficiency in calcium and magnesium within stock, leading to milk fever or grass tetany, respectively. It must be noted that there are various grazing management practices that can be followed to minimise the risk and being mindful of the animal’s demand for Ca and Mg, such as prior to calving and early lactation. Studies completed by Gourley 1999 and Hosking 1986 have recommended to not exceed 120kg K/ha annually and 60 kg K/ha in one application, to reduce this risk.
The peak demand for potassium increases when plants are actively growing. Potassium can be supplied in combination with nitrogen and other nutrients to boost growth and dry matter production. In this situation, boosting growth from nitrogen and to get the best nitrogen use efficiency, rates should be based on supplying 25-60 kg/ha of nitrogen. Next consider any phosphorus and potassium required for capital applications to build soil fertility, then the maintenance rates needed to replace removal.
In Conclusion
Potassium management is complex, and there are a range of variables that affect its availability for plant uptake including removal rates, soil texture, and plant physiology (root depth).
Topsoil testing, segmenting at depth, and plant tissue tests are tools that can assist in supporting a potassium recommendation.
If an application is needed to either maintain or build (capital) K level, product selection, timing, and rate need to be carefully considered to reduce possible seedling damage, and/or animal health issues.
Further information
For more information, feel free to contact me at fiona.mcdonald@incitecpivot.com.au or +61 488 772 144.
References
Murrell, RL Mikkelsen, G Sulewski, R Norton, ML Thompson (2021) ‘Improving Potassium Recommendations for Agricultural Crops(eBook)’ (Springer: Switzerland) https://doi.org/10.1007/978-3-030-59197-7
Witter E, Johansson G (2001) Potassium uptake from the subsoil by green manure crops. Biol Agric Hortic 19:127–141. https://doi.org/10.1080/01448765.2001.9754917
Hosking WJ (1986) Potassium for Victorian Pastures—A Review. Department of Agriculture and Rural Affairs, Victoria.
Gourley CJP (1999) Potassium. In ‘Soil analysis: an interpretation manual’. (Eds KI Peverill, LA Sparrow, DJ Reuter) pp. 229–239. (CSIRO Publishing: Melbourne, Vic.)
Disclaimer ® EASY N and Nutrient Advantage are registered trademarks of Incitec Pivot Fertilisers.
DISCLAIMER
This is a guide only, which we hope you find useful as a general tool. While IPF has taken all reasonable care in the preparation of this guide, it should not be relied on as a substitute for tailored professional advice and IPF accepts no liability in connection with this guide. Incitec Pivot Fertilisers manufactures and sources fertilisers from other suppliers. The fertiliser supply chain extends beyond the company’s direct control, both overseas and within Australia. Incitec Pivot Fertilisers hereby expressly disclaims liability to any person, property or thing in respect of any of the consequences of anything done or omitted to be done by any person in reliance, whether wholly or in part, upon the whole or any part of the contents of this article.
You might also be interested in these

Winter Crop
Flexible phosphorous strategies in canola to optimise establishment
April / 2023

Horticulture, Pasture, Sugar, Summer Crop
Poor Soil Structure – Have you identified if it is costing you profitability?
November / 2023

Pasture
Dedicate time for fertiliser planning
March / 2025

Summer Crop
Know your Zinc application options
November / 2024

