Agronomic Insights

Soil testing to establish the nutrient status of your fields is an important action for the coming months. Nutrient removal over the last three years has been high due to high-yielding crops and/or losses from denitrification, erosion and leaching. This means the rules used by agronomists and growers to calculate nutrient application rates may not be relevant for the coming season.
David McRae – IPF Technical Agronomist
Mobile nutrients, such as nitrate nitrogen and sulphate sulphur, will have leached down the soil profile because of the increased quantities of water moving down.
In 2021 at the IPF “Colonsay” long-term field trial site on the central Darling Downs, deep drainage was calculated using the How Wet Module – Australia CliMate.
For the fallow period after wheat was harvested in 2021, 292 mm of deep drainage occurred. This would have contributed significantly to nitrogen losses at this site, discussed in a recent Agronomic Insights article.
Location of sampling points
After significant wet periods, it can be worthwhile rethinking your usual sampling.
Fields may have experienced different conditions depending on their topography and drainage e.g., long, short or no periods of flooding and waterlogging. So, previous sampling plans may no longer be relevant and may result in misleading or incomplete information on which to base your next fertiliser program. Consider sampling affected areas separately to capture the accurate nutrient baseline and establish an appropriate fertiliser rate.
Soil collection is the first step and has the greatest influence on the reliability of a soil test result. Care and attention must be taken during this process to ensure samples are collected, handled, and subsampled correctly. Results of soil testing are only as good as the method and location of the sample itself.
Sample segmentation
Understanding both the amount and depth of available nutrients in the soil profile is important as it has a large bearing on when the crop will be able to access those nutrients.
Nutrient requirements change as plant development progresses. To optimise yields, nutrients need to be available at the right times to satisfy crop demand.
Crop species vary in their ability to take up nutrients from soils. Root density changes as they grow deeper in the soil profile. Figure 1 illustrates the difference in root density for 7 field crops. As can be seen, there is a large variation in the quantity of root in the top 50% of the rooting depth. Crops with higher root densities will be able to take up more nutrient than those with lower densities. This is particularly important for uptake of immobile or poorly mobile nutrients such as phosphorus, potassium and zinc as roots need to grow towards and intercept these nutrients.
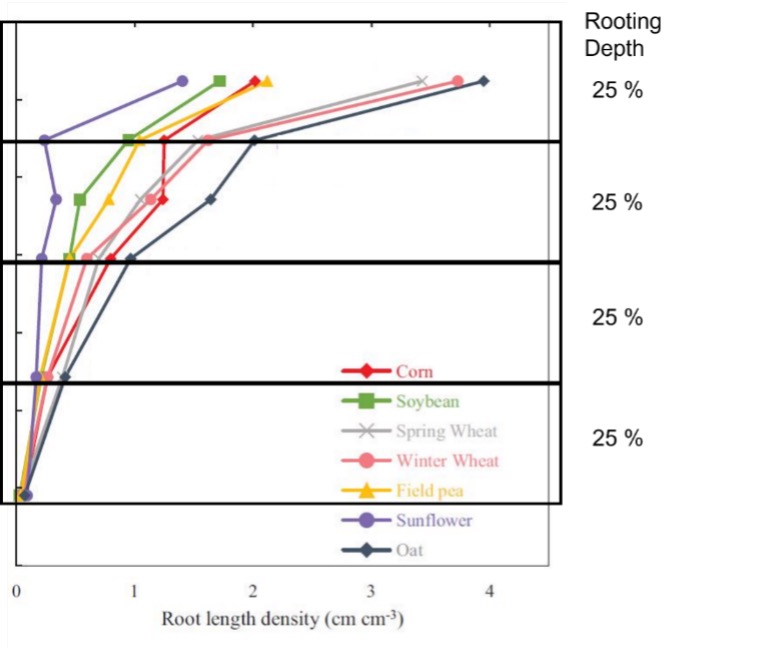
Another consideration is the speed at which roots grow and access nutrients. For example, wheat will generally have developed a root system to 60 cm of depth (approx. 50% of rooting depth) by the end of tillering without subsoil constraints. Therefore, the top 60 cm will need to contain at least enough nutrients (soil + fertiliser) to satisfy crop requirements to this crop stage. Nutrients such as nitrogen and sulphur will become available if the crop needs to access moisture deeper than 60 cm, otherwise nutrients below this depth will only have a small contribution to the crop requirements.
Considerations when segmenting your deep soil samples
Generally, deep soil nitrogen samples are taken to a depth of either 80 cm or 90 cm, and the question of how best to segment samples is often asked.
Some points to consider include the uniformity of nitrogen and sulphur as soil depth increases. Distribution down the profile will affect how and when the crop accesses these nutrients. Even distribution of nutrients down the profile will improve uptake, but profiles with uneven nutrient distribution may reduce crop growth due to bands of low nutrient availability.
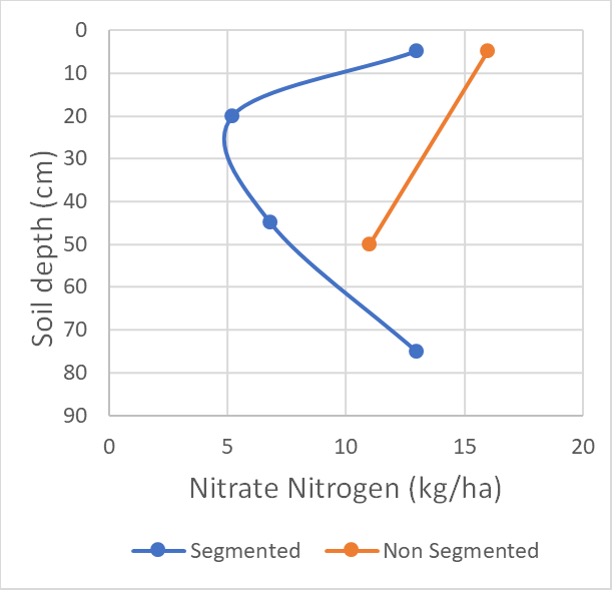
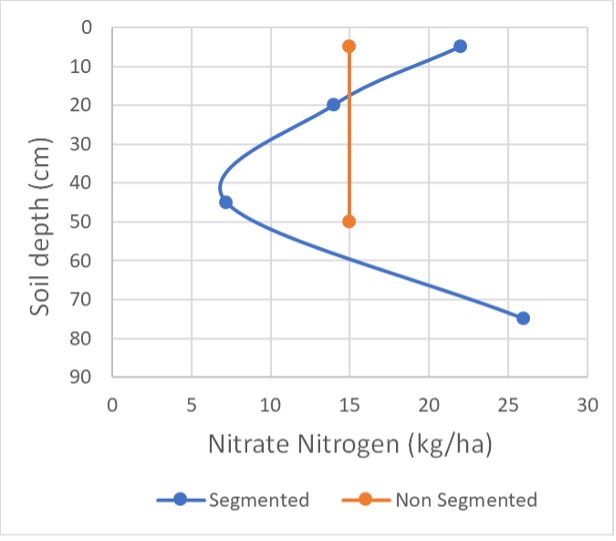
To investigate this topic, random samples were taken from different nitrogen rate plots at the “Colonsay” site. Two sampling strategies were used:
1. segmented – 0-10cm, 10-30cm, 30-60 cm & 60-90 cm
2. non-segmented 0-10 cm & 10-90 cm
Profile calculations for N & S for each sampling strategy varied up to 25 kg/ha for N and 80 kg/ha for S. The smaller variation for N is probably not significant in terms of the total N figure but of more concern is the variation down the profile. In both Figures 2 & 3, if you just used the non-segmented strategy, N appears to be relatively well distributed down the profile.
However, when it is compared to the segmented strategy, both plots have lower N availability in the 10-60 cm layers. This may change your interpretation of the results and need for N or the timing of application. Figure 2 has approximately 160 kg/ha N available, but the 30-60 cm layer is quite depleted. If you had not identified the lower availability of N in this layer, crop development leading to fully tillered / stem elongation stage would probably be suffering from low N availability.
Pages 21-22 of the Fertcare guide “A guide for ‘fit for purpose’ soil sampling” provides good information on correct sampling depths (Fertcare soil sampling guide).
Selecting the appropriate sampling sites and correct handling of samples will provide the best results for developing this season’s fertiliser program. With abnormal conditions over the last few years, identifying soil nutritional status is very important.
Plant nutritional status can be monitored throughout crop development with plant tissue analysis and grain analysis at harvest.
Further Information
David McRae on 0477 987 321 david.mcrae@incitecpivot.com.au
Bede O’Mara on 0417 896 377 bede.omara@incitecpivot.com.au
Jim Laycock on 0427 006 047 jim.laycock@incitecpivot.com.au
References
Australian CliMate website (https://climateapp.net.au/)
Osborne, S.L., Chim, B.K., Riedell, W.E. & Thomas E. Schumacher (2020). Root length density of cereal and grain legume crops grown in diverse rotations. Crop Science. 2020; 60: 2611–2620.
Resources
Download InsightDISCLAIMER
This is a guide only, which we hope you find useful as a general tool. While IPF has taken all reasonable care in the preparation of this guide, it should not be relied on as a substitute for tailored professional advice and IPF accepts no liability in connection with this guide. Incitec Pivot Fertilisers manufactures and sources fertilisers from other suppliers. The fertiliser supply chain extends beyond the company’s direct control, both overseas and within Australia. Incitec Pivot Fertilisers hereby expressly disclaims liability to any person, property or thing in respect of any of the consequences of anything done or omitted to be done by any person in reliance, whether wholly or in part, upon the whole or any part of the contents of this article.





